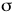 bonds (or atoms) around the central atom.
bonds (or atoms) around the central atom.VSEPR: Valence Shell Electron Pair Repulsion theory, allows one to predict molecular structure. If molecular structure can be predicted then molecular properties, like polarity, can be predicted.
To predict the structure of a molecule we simply need to arrange the pairs of electrons to minimize the repulsion between them.
1. First determine the steric number by counting the number lone pairs and 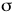 bonds (or atoms) around the central atom.
bonds (or atoms) around the central atom.
2. The pairs of electrons must be distributed in space to minimize pair replusions. However, it is important to note that lone pairs take up more space than bonding pairs.
3. The positions of the atoms in the molecule are used to name the shape of the molecule.
|
lone pairs + |
|
geometry |
|
|
|
linear |
linear |
|
|
|
linear, |
linear |
|
|
|
trigonal planar, |
trigonal planar V-shaped |
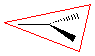 |
|
|
tetrahedral, |
tetrahedral pyramidal bent |
 |
|
|
trigonal bipyramid, |
trigonal bipyramid see saw T-shaped linear |
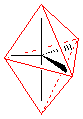 |
|
|
octahedral, 90° apart |
octahedral square pyramidal square planar T-shaped linear |
 |
|
|
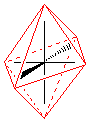
pentagonal bipyramidal |
|
 |
|
|
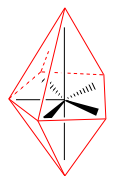
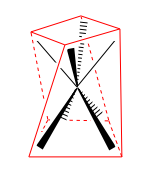
square antiprismatic |
|
 |