 H =
H =
 E +
E +
 P
P V
VEnthalpy is defined as follows
Enthalpy is the sum of the internal energy
and pressure times volume.
We cannot measure the enthalpy of a system, but we can look at changes in enthalpy.
 H =
H =
 E +
E +
 P
P V
Vto make life easier we will make certain that Pressure is held constant...
 H =
H =
 E +
P
E +
P V
VSo, the change in enthalpy,  H,
is equal to the change in internal energy,
H,
is equal to the change in internal energy,  E,
plus PV work, P
E,
plus PV work, P V.
V.
The expression for  H
can be simplified.
H
can be simplified.
Since,
 E = q +
w
E = q +
wand work is - P V
V
 E =
qp - P
E =
qp - P V
Vq is given the subscript "p"
because
we are holding the pressure constant.
Substituting for  E
in
E
in  H =
H =
 E +
P
E +
P V
gives
V
gives
 H =
qp - P
H =
qp - P V
+ P
V
+ P V
VSo, at constant pressure allowing for only PV work (no other kind of work occurring)
 H =
qp
H =
qp
DH < 0 then qp is negative which means heat is leaving the system. A process which releases heat is exothermic.
DH > 0 then qp is positive which means heat is being absorbed by the system. A process which absorbs heat is endothermic.
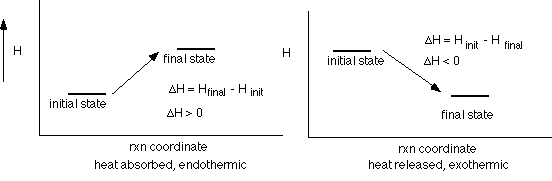
Remember this does not represent the change in the total energy content (internal energy, E) it just tells us how much heat energy is released during a change occuring under very specific conditions.