 T
TWhat is heat? Is heat temperature? No!
Which would be worse, putting you finger in a jigger of boiling water or putting you finger in a pot of boiling water?
Why would it be worse to put your finger in the pot of water; after all, the water in each container is at the same temperature?
Since there is more water in the pot, the pot has the ability to transfer more heat to your finger, and thus do more damage.
The jigger of boiling water releases heat to your finger. Since your finger contains a good deal of water all the energy is absorbed, and the temperature of you finger goes up a little.The pot of boiling water releases heat to your finger. All the heat of the boiling water (if you can keep your finger in the water that long) is absorbed by your poor tiny little finger, and the temperature goes up a lot!
 T
TSo, a certain amount of heat can change the temperature of an object, but the size of the temperature change does not simply depend on the amount of heat; the size of the temperature change also depends on the identity of the object.
 T
TC is the amount of heat (q) required to raise the temperature of an object by 1 K (T). The units would be J/K.
The heat capacity of an object depends on
For objects of uniform composition; i.e., objects that are the same all the way through, it is convenient to break down C into its components, size and identity.
Identity, what do you mean?Water is water, and it will always take the same amount of heat to increase the temperature of 1 g of water by 1 K.
The same thing can be said from a molar point of view; it will always take the same amount of heat to increase the temperature of 1 mol of water by 1 K.
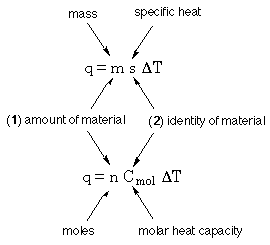
The amount and identity statement can be expressed in terms of grams and specific heat, or
moles and molar heat capacity.
 T TWhen discussing heat in terms of grams of material the Specific Heat, s, is the quantity that is used. Specific heat is the flow of heat required to change the temperature of 1 g of a substance by 1 K.
The unit for s (specific heat) is |
 T TWhen discussing heat in term of moles of material the molar heat capacity, Cmol, is the quantity that is used . The molar heat capacity is similar to specific heat, but instead of heat flow per g the number is heat flow per mole. The molar heat capacity is the heat flow required to change the temperature of one mole of a substance by 1 K. The unit for Cmol is |