Many alternative automobile fuels have been suggested. Methanol is one of them. There is an interesting way to to boost the amount of energy that can be extracted from a methanol powered engine. A catalyst can convert methanol to formaldehyde and hydrogen.
Experiments were performed to determine the heat of combustion of methanol, formaldehyde, and hydrogen.
![]()
![]()
![]()
Using this data determine the DHrxn for the conversion of methanol to formaldehyde and hydrogen.
Rearrange the equations so that they add up to the reaction of interest.
Methanol is a reactant so write the reaction,
![]()
But we only need 1 methanol molecule, so cut the reaction in half...
![]()
We have to divide EVERYTHING including the DH. Since we are only doing half the reaction we only get half the energy.
We need H2 as a product so the reaction must be turned around.
![]()
If the reaction is turned around then the energy must be turned around too. The forward reaction released energy; to get the reaction to go backwards energy must be absorbed by the reaction.
![]()
Once again we only need one H2; so , divide by 2.
![]()
Formaldehyde is also a product; so, its combustion reaction must be turned around. Since the reaction is being turned around, the sign of DH must be changed.
![]()
Let's add it up to see if we get the reaction we want.
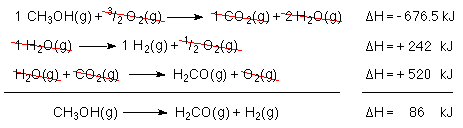
By converting the methanol to formaldehyde and hydrogen the enthalpy of the fuel has been increased. When the formaldehyde and the hydrogen are burned 86 kJ more energy will be released than when methonol is burned!
 H's here are given as heats
of reaction. However, if someone were to announce that the heat of
combustion of methanol is -677 kJ the
H's here are given as heats
of reaction. However, if someone were to announce that the heat of
combustion of methanol is -677 kJ the  H
is per mole.
H
is per mole.
For example the statement:
 Hcombustion = -677 kJ
for methanol
Hcombustion = -677 kJ
for methanolmeans that for each mole of methanol burned 677 kJ heat are released.