If the  H of a reaction is known, then
heat can be treated as though it were a reactant or a product; that
is, the amount of heat absorbed or released can be related to the
amount of material produced or consumed during a reaction by using
the stoichiometry of the reaction.
H of a reaction is known, then
heat can be treated as though it were a reactant or a product; that
is, the amount of heat absorbed or released can be related to the
amount of material produced or consumed during a reaction by using
the stoichiometry of the reaction.
Someone determined that burning a mole of CH3OH
releases 676.5 kJ of heat; this is usually written  Hcombustion
= -676.5 kJ/mol for CH3OH.
Hcombustion
= -676.5 kJ/mol for CH3OH.
How much heat energy is released when 5.8 g methanol are
burned?
The balanced reaction is:
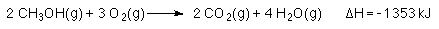
Well actually, the question is, if 2 mol
CH3OH of produces 1353 kJ of heat then how
many kJ are produced when 5.8 g of CH3OH are burned.
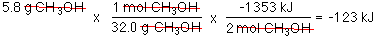
 H =
-123 kJ
H =
-123 kJ
It is important to note that  H = -123
kJ, when asked how much heat is released by the reaction the the
response is
H = -123
kJ, when asked how much heat is released by the reaction the the
response is
123 kJ of heat are released,
not -123 kJ of heat are released.
The statement "-123 kJ of heat are released" would actually mean
heat was being absorbed.
This is the energy released as heat. Remember the reaction creates
gases, and the gases expand into the room/container, so there is work
being done by the reaction. That is, some of the energy of the
reaction goes into expanding the gases which are produced and when we
measure or discuss  H we are ignoring the
energy which is used to expand the gas.
H we are ignoring the
energy which is used to expand the gas.
 H of a reaction is known, then
heat can be treated as though it were a reactant or a product; that
is, the amount of heat absorbed or released can be related to the
amount of material produced or consumed during a reaction by using
the stoichiometry of the reaction.
H of a reaction is known, then
heat can be treated as though it were a reactant or a product; that
is, the amount of heat absorbed or released can be related to the
amount of material produced or consumed during a reaction by using
the stoichiometry of the reaction.