Acid-Base Titration reactions
Titration reactions are just neutralization reactions. Titrations are used to determine the amount of one substance present by reacting it with a known amount of another substance.
For instance, you can find the molar mass of an acid by titrating the acid with a solution of base of known concentration.
e.g.
What is the molecular weight of an unknown monoprotic acid if 0.4955 g of the acid are neutralized by 37.00 mL of a 0.1000 M NaOH solution?
How do you answer this question? If you want to find the molar mass you need to know how many grams of acid are in 1 mole, or if you knew how many moles were in the sample above then you could calculate the molecular mass
So how many moles of acid are there?
That is OK; we know how many protons the acid has.
![]()
The "?" is some unknown ion. After all an acid is just a proton, an H+, and an anion.
The number of moles of acid can be related to the number of moles of base using the balanced equation.
Remember, we know the mass of the sample; so, all we need to do is find the number of moles of acid are in the sample.
Where to start?
Starting with g of acid leads nowhere....
![]()
![]()
To find the moles of acid
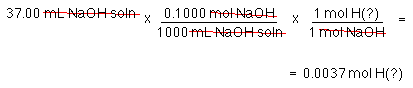
Remember, number of moles of acid is not the answer. You must find molar mass which is grams per mole.
Titrations are really stoichiometry problems, but the formula of the acid is unknown.