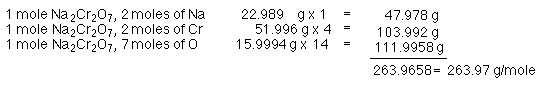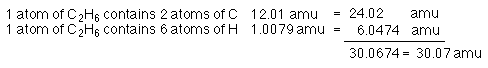
Chemical Formulae
Chemical formulae are used as shorthand to indicate how many atoms of one element combine with another element to form a compound.
C2H6, 2 atoms of carbon combine with 6 atoms of hydrogen to form ethane.
Na2Cr2O7, two Na combine with 2 Cr and 7 O to form sodium dichromate.
If the formula of a compound is known determining the molar mass
is simple. Since we know the atomic masses we can sum the atomic
masses to obtain the molecular mass of any molecule.
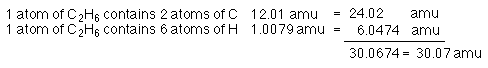
We also know that 1 mole of C atoms weighs 12.01 g so we know the
molar or mass of ethane also.
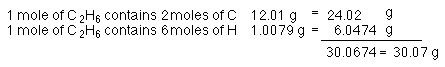
Or, 30.07 g in 1 mole C2H6. So, we say the
molar mass of ethane is 30.07 g/mol (that's grams per mole).
Determine the molar mass of Na2Cr2O7