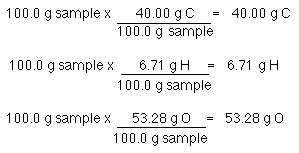
Why use 100 g?
Because it is convenient.
Percent Compostition
Why do we care about % composition?
When an elemental analysis is performed we do not get the molecular formula. The result of an elemental analysis is always reported as a percent mass of each of the elements for which the analysis was performed.
An elemental analysis of a white crystalline compound believed to
be C6H12O6, analyzed for C, H and O
gave the following results.
Is the the compound actually C6H12O6?
The problem is really just a conversion from % by wt. to moles. Why a conversion to moles? Because that is what a formula is, a formula relates moles of one element to moles of another element.
Start the problem with the assumption that you have 100.0 g of sample. The percent composition tells you how much of each element is present:
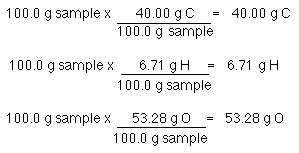
Why use 100 g?
Because it is convenient.
The formula is determined by the mole ratio of the elements, not the mass ratios. So, we need to convert the number of grams to number of moles.
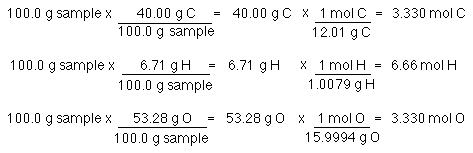
Finally, the mole ratio is determined. Divide each molar amount by the smallest molar amount. The ratio is
According to our calculations we have CH2O or formaldehyde (which is a gas)!
Are we wrong...NO! We have determined the ratios in which the
elements are combined to form the compound, but not the actual amount
of the elements present. To determine the actual amount of each
element present we need to know the Molar Mass (often refered to as
the molecular weight or MW).
The molar mass must be determined using another experiment (A mass
spectrometer could determine the molar mass. There are other less
exspensive ways to determine molar mass, and we will discuss some of
them next semester.)
To determine the molecular formula we will use the empirical formula
which we just found. The molecule must be made up of some number
of empirical formula units; that way the ratio of the elements
remains the same:
If we knew any two of these numbers then we could find the third,
but we only know one. If the molecule is made of some number of
empirical formula units the the molar mass must be some multiple of
the empirical mass.
Since, empirical mass CH2O = 30.25 g/mole
Essentially, we found how many empirical formula units are in the whole.
A bottle of improperly labeled "iron oxide" is found in a laboratory. Elemental analysis reveals that the sample is iron and oxygen, and the sample is 69.94 % iron by mass. What is the formula of the material, and what should the bottle be labeled; that is, what is the name of the compound?
To find the formula we need to know the % mass of each of the elements. Since, there are only 2 different elements in iron oxide we know...
So,
Now that we have % mass of both elements let's find the formula. Once again assume a100 g sample.
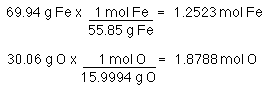
Reduce to lowest ratio by dividing each number by the smallest number...
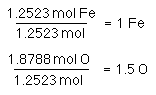
Formulae do not (usually) contain fractions. So, we must find the lowest common whole number multiple.
So, multiply each number by 2
So, the formula is
The name is
To find a formula we do not need to have % composition.
If we are told the mass of each element present in a compound we can find the formula. The mass of the elements can be converted to moles of the elements. The mole ratio reveals the empirical formula.
For example,
A compound containing only Mn and Cl contains 1.9228 g Mn and 2.4817 g Cl. Determine the empirical formula.
We simply need to convert the masses of the elements to moles. The empirical formula is determined by the mole ratio of Mn to Cl.
The formula is
Combustion analysis is used to determine the empirical formula of hydrocarbons (a compound containing only hydrogen and carbon).
Combustion of a hydrocarbon with a molar mass of 78.11 g/mol produced 2.6406 g CO2 and 0.5400 g H2O. Determine the molecular formula.
Since we do not know the formula we cannot balance the equation.
The important thing to note is that all the carbon in the CO2 must come from the hydrocarbon. All the hydrogen in the water must also come from the hydrocarbon!
If we determine the number of moles of C in the CO2 we will have the number of moles of carbon in the hydrocarbon. If we determine the number of moles of H in the H2O we have the number of moles of H in the hydrocarbon.
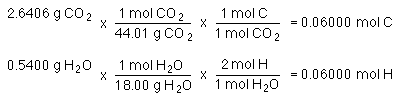
The empirical formula is
So, the molecular formula is made up of some number of empirical formula units. Use the molar mass and the empirical formula mass to determine that number.
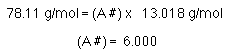
The molecule contains 6 empirical formula units. The molecular formula is