|
|
|
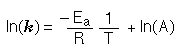 |
It does not take a leap of faith to realize that molecules must collide for a reaction to occur. However, not every collision produces a reaction; if every collision were successful all reactions would be completed almost immediately.
A theory to explain the was developed by Svant Arrhenius rate of reactions. For a collision to be successful in producing a reaction it must meet some requirements. These special conditions are that (1) the molecules must collide at the right angle so the orbitals can interact, and (2) the molecules must have enough energy so the orbitals and the electrons can be rearranged.
It is not hard to accept that the number of molecules with sufficient energy increases with temperature. As temperature increases the speed of the molecules increases; therefore, the energy of the collisions increases. More often than not, the increase in energy causes an increase in the rate of the reaction. Since the rate of a reaction inreases with increasing temperature there must be a relationship between the rate constant and temperature.
The relationship can be expressed using either of the following equations:
|
|
|
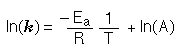 |
The rate constant is related to temperature through the activation energy, Ea. The activation energy is the energy barrier which must be overcome for the reaction to proceed.
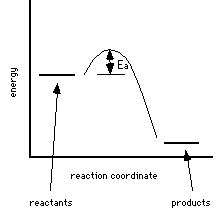
1 |
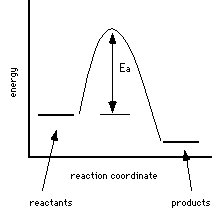
2 |
Both reactions 1 and 2 release energy; reaction 1 releases more energy than reaction 2. Reaction 1 occurs more quickly than reaction 2; the activation energy is lower for reaction 1. Since the Ea is lower more reactant molecules can overcome the barrier and the reaction proceeds more quickly than reaction 2.
|
determining |
go back to Gen
Chem 2
Home Page