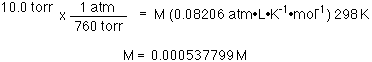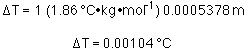Osmosis: Two samples of water are
separated by a semipermeable membrane. Water flows back and forth,
but no solute molecules can flow through the membrane.
If one side contains solute particles then on that side there are
fewer water molecules next to the membrane, which means that there
are fewer water molecules to go through the membrane. The solvent on
the side without solute particles diffuses through the membrane more
quickly than the side with solute particles present.
|
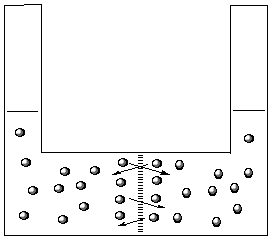
In the drawing to the left, water molecules can pass
freely through the membrane. Since the same number of
molecules move in each direction the volumes do not
change.
|
|
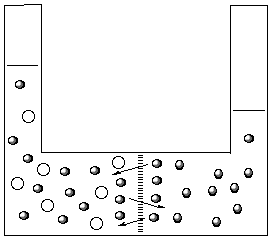
Now that there are solute particles next to the membrane
there are fewer water molecules to pass through the
membrane. Since the more water moves from the right to the
left than from the left to the right the volume increases on
the left hand side. The volume keeps increasing until the
pressure caused be the height of the column of water is
large enough to counterbalance the influx of water from the
right side.
|
The relationship between osmotic pressure and concentration is
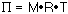
Where  is the osmotic pressure, the pressure required to prevent the flow of
solvent from one side of the membrane to the other, in atm, M is the
concentration of the solution in moles per liter, R is the universal
gas constant 0.08206
L•atm•mol-1•K-1, and T is the
absolute temperature of the solution.
is the osmotic pressure, the pressure required to prevent the flow of
solvent from one side of the membrane to the other, in atm, M is the
concentration of the solution in moles per liter, R is the universal
gas constant 0.08206
L•atm•mol-1•K-1, and T is the
absolute temperature of the solution.
For example
35.0 g of hemoglobin are dissolved in water to make a 1 L
solution. (I do not know anyone who would do this experiment on such
a large scale. It would make more sense to make ca. 1 mL, or less, of
the solution.) At 25 °C the osmotic pressure was found to be
10.0 torr. What is the molecular mass of hemoglobin?
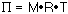
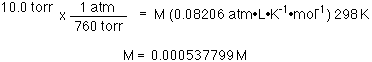
The 1 L solution contains 0.000537799 mol or 35.0 g so...
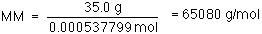
Our least precise measurement has three significant figures (35.0
g, 10.0 torr and 273+25 = 298 K) so our answer can have three
significant figures.
MM = 65,100 g/mol
Because we used a more sensitive property, osmotic pressure, we
arrived at a more precise molar mass determination than BP elevation
or MP depression could provide. In fact, BP elevation and MP
depression would not work for hemoglobin. The temperature change
would be to small.
What kind of freezing point depression would one see? Because the
solution is so dilute the molality is almost the same as the
molarity. So, let us assume that m = M, or m = 0.0005378
m.
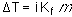
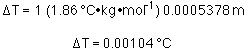
Essentially, freezing point depression or boiling point elevation
would not be measureable.