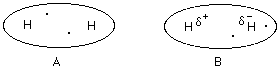
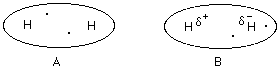
On average an H2 molecule resembles molecule A; the electrons are distributed evenly.
However, the electrons could be distributed unevenly for a moment in time. Since the electrons are on one side of the molecule there is a positive side and a negative side to H2 molecule B.
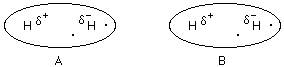
The positive side of the polar H2 molecule B attracts the electrons of H2 molecule A.
A dipole-dipole interaction is now occuring.