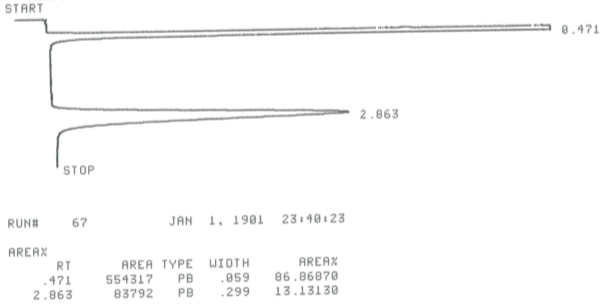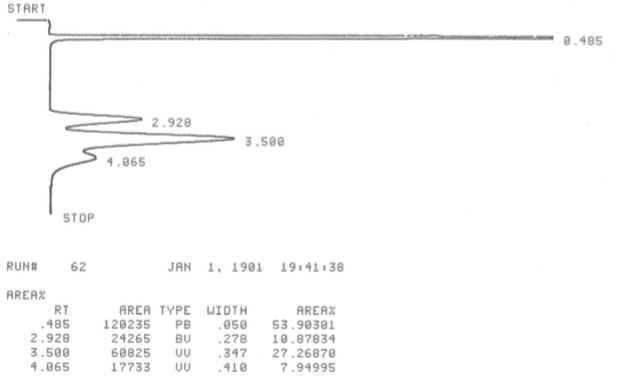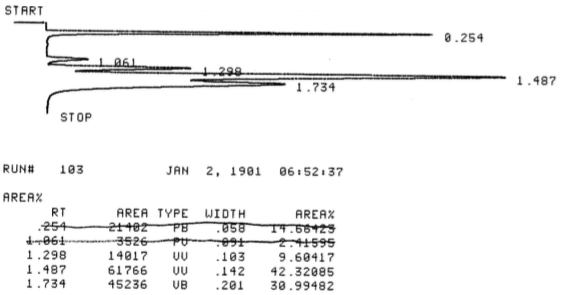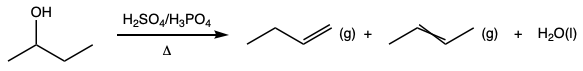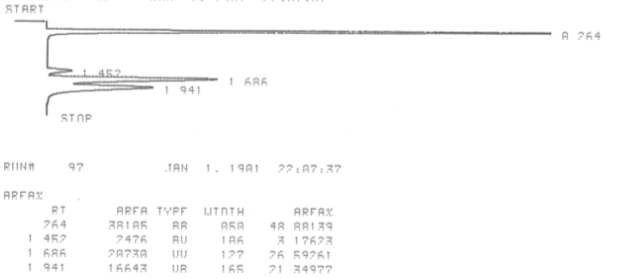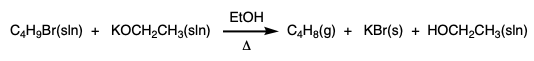

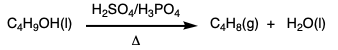
During this activity students investigate E2 and E1 reactions by reacting 1- and 2-bromobutanes with ethoxide and by reacting 1- and 2-butanol with sulfuric and phosphoric acid. The product distributions are investigating by examining chromatograms collect from gas chromatographic analysis of the gaseous products.

|
A solution of potassium ethoxide in ethanol (3 mL) is added to a 5-mL conical vial.
|
|
|
1-Bromobutane (0.32 mL) is added to the 5-mL vial. The ground glass joint on the gas-delivery tube is greased with silicone grease and connected to the vial. |
|
|
The apparatus is inserted into an aluminum block that has already been heated to 80 to 90 °C, an previously prepared, water-filled, inverted test tube is placed over the gas-delivery tube, and the gas being pushed out of the 5-mL vial is collected. A after a few moments bubbles can be seen collecting in the inverted test tube. |
|
|
After collecting approximately 4 mL of gas, the test tube is removed and a gas collection tube capped with a septum is placed at the end of the gas-delivery tube. |
|
|
After collecting approximately several milliliters of gas, a syringe is used to collect a sample of gas for analysis on a gas chromatograph. |
|