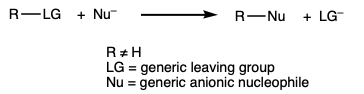

During this activity students investigate the solubility and reactivity of n- and t- pentanol in water and hydrochloric acid.
|
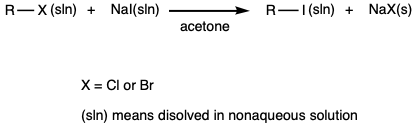
A solution (1 mL) of sodium iodide dissolved in acetone is added to eight test tubes. Sodium iodide is soluble in acetone whereas sodium chloride and sodium bromide are not. Thus, the formation of a precipitate is evidence that the iodide displaced the leaving group, which then precipitated from solution. |
|
|
A few drops of the following alkyl halides are added, one compound for one of each of the test tubes, to the first four test tubes.
1-chlorobutane |
|
|
A few drops of the following halohydrocarbons are added, one compound for one of each of the test tubes, to the second four test tubes.
2-chloro-2-methylpropane |
Note: The test tube labeled 2-chloro-2-methylbutane is mislabeled. 2-Chloro-2-methylpropane is the chemical that was added to the reaction. |
|
The mixtures that remained clear are transfered to a warm water bath (50 °C max). |
Note: The test tube labeled 2-chloro-2-methylbutane is still mislabeled. 2-Chloro-2-methylpropane is the chemical that was added to the reaction. |
|
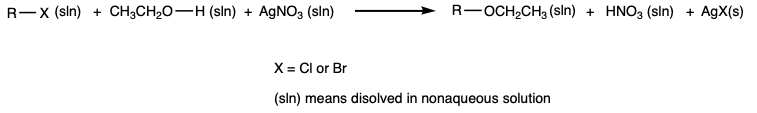
A solution (1 mL) of silver nitrate dissolved in ethanol is added to eight test tubes. Silver nitrate is soluble in ethanol, but silver chloride and silver bromide are not. The formation of a precipitate is evidence that a nucleophile (ethanol, which is also the solvent) has displaced the halogen leaving group. |
|
|
A few drops of the following alkyl halides are added, one compound for one of each of the test tubes, to the first four test tubes.
1-chlorobutane |
|
|
A few drops of the following halohydrocarbons are added, one compound for one of each of the test tubes, to the second four test tubes.
2-chloro-2-methylpropane |
Note: The test tube labeled 2-chloro-2-methylbutane is once again mislabeled. 2-Chloro-2-methylpropane is the chemical that was added to the reaction. |
|
The mixtures that remaineded clear are transfered to a warm water bath (80 °C max). |
Note: The test tube labeled 2-chloro-2-methylbutane is still mislabeled. 2-Chloro-2-methylpropane is the chemical that was added to the reaction. |