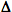 H,
H, 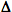 S
SGoals 4.1: Thermodynamics: 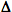 H,
H, 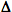 S
S
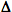 H and
H and 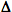 S and use their signs to determine whether enthalpy and entropy are increasing or decreasing
S and use their signs to determine whether enthalpy and entropy are increasing or decreasing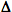 Suniverse and
Suniverse and 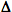 Ssystem
SsystemGoals 4.2: Thermodynamics: 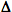 G, and K
G, and K
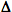 G and
G and 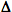 Suniverse
Suniverse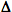 G°,
G°, 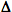 G°' and
G°' and 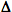 G
G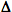 G and K to determine one when the other is given
G and K to determine one when the other is givenGoals 4.3: 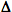 G, using favorable reaction to drive unfavorable reactions, and the role of ATP in energy transfers
G, using favorable reaction to drive unfavorable reactions, and the role of ATP in energy transfers
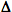 G to describe how one reaction can be used to drive another
G to describe how one reaction can be used to drive another