What is the density of CH4 at 25 °C and 1 atm? How about Xe?
Find the mass of CH4 in 1 cm3
That is, find the mass and the volume of 1 mole of CH4 and you will have found its density!
So, what is the volume that 1 mole of methane occupies?
V = 24.47 L
density equals mass divided by volume...
1 mole of Xe will occupy the same volume as 1 mole of methane; after all, the ideal gas law does not have a term (variable) for kind of gas, just amount of gas!
so, the density of Xe is
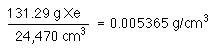
A simple experiment can be performed to determine the molar mass of an unknown volatile liquid. 1.5021 g of a volatile liquid was placed in a container which had a small pin-hole in the top, and the container was placed in boiling water. After all of the liquid had vaporized and the vapor stopped escaping from the pin-hole the container was cooled back to room temperature. 0.7523 g of the volatile liquid remained in the container, the volume of the container is 0.500 L, and the atmospheric pressure on that day was 756 torr. Determine the molar mass of this compound.
To determine the molar mass the number of moles in a given mass needs to be determined.
So, the question we have to answer is how many moles of liquid are present in a sample of liquid.
The key here is that at one point in the experiment all of the liquid was converted to gas."After all of the liquid had vaporized the container was cooled back to room temperature. 0.7523 g of the volatile liquid remained in the container"So, we have alot of information about the unknown.
We know
So,
We know P, V, and T! We can find n.
n = 0.016242 mol
So, g per mole