Gas Laws and Kinetic Molecular Theory
The Gas Laws are based on experiments, and they descibe how a gas
behaves under certain conditions. However, the ideal Gas Law does not
attempt to explain the behavoir of gases. A theory must be developed
to explain the behavoir of gases.
The Kinetic Molecular Theory is a model that explains some of the
behavior of gases. The Kinetic Molecular Theory relies on some
assumptions that are shown to be reasonable through
experimentation.
We will not derive the KMT. However, we could show that the following statement is reasonable.
The pressure exerted by a single particle in a container is
Because the velocity of all the gas particles is different, the pressure exerted by a bunch of molecules is
Since the mass of the gas particles does not change lets factor out the "1/3 m".
To make this expression applicable to a container filled with any number of gas particles we will find the average velocity squared and then multiply by the number of particles.
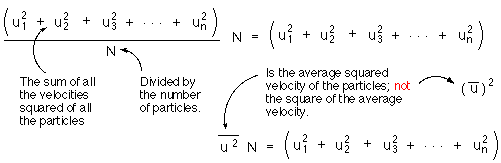
Substitute ![]() for
the sum of the squared velocities.
for
the sum of the squared velocities.
remember N is just the number of particles
Remember that PR = nRT and substitute nRT for PV in the equation above.
T is proportional to ![]() .
That is,
.
That is,
molecular motion increases with increasing temperature.
Since,
and
![]()
Since the number of particles can be expessed as number of moles
times Avagadro's number, ![]() .
.
![]()