Precipitation reactions
Not all ionic compounds are soluble in water. If PbSO4
is added to water, none of the lead(II)sulfate will dissolve. The
interaction between Pb2+ and SO42-
is simply stronger than the attraction of the water to either the
Pb2+ or SO42- (Both
enthalpy and entropy play a roll. We will discuss entropy and lattice
energy second semester. We will discuss enthalpy and bond energy
later this semester).
Simple Rules for the Solubility of Salts in Water
- Most nitrate (NO3-) salts are
soluble.
- Most salts containing the alkali metal ions (Li+,
Na+, K+, Cs+, Rb+) and
the ammonium ion (NH4+) are soluble.
- Most chloride, bromide and iodide salts are soluble. Notable
exceptions are salts containing the ions Ag+,
Pb2+, and Hg22+.
- Most sulfate salts are soluble. Notable exceptions are
BaSO4, PbSO4, HgSO4, and
CaSO4.
- Most hydroxide salts are only slightly soluble. The important
soluble hydroxides are NaOH and KOH. The compounds
Ba(OH)2, Sr(OH)2, and Ca(OH)2 are
marginally soluble.
- Most sulfide (S-2), carbonate
(CO32-), chromate
(CrO42-), and phosphate
(PO4-3) are only slightly soluble.
If two solutions are mixed together it is possible that two ions
could combine to form an insoluble ionic complex.
A solution of silver nitrate is combined with a solution of sodium
chloride. The resulting solution contains Na+,
Ag+, Cl-, and NO3-, but
AgCl is not soluble in water. Since Ag+ is now in solution
with Cl- the two will combine to form AgCl, and the AgCl
will precipitate from solution.
The reaction can be described in a number of ways
- A "molecular " equation could be used:
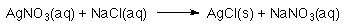
- A "complete ionic " equation could be used:
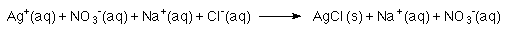
- A "net ionic " equation could be used.
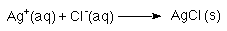
Net ionic equations are found by writing the full equation and
then eliminating the spectator ions; spectator ions are ions that
do not participate in the reaction. In the example above
Na+(aq) and NO3- are present as
both products and reactants. Since Na+(aq) and
NO3- do not participate in the reaction they
are called spectator ions.
return to GenChem Home
Page
![]()