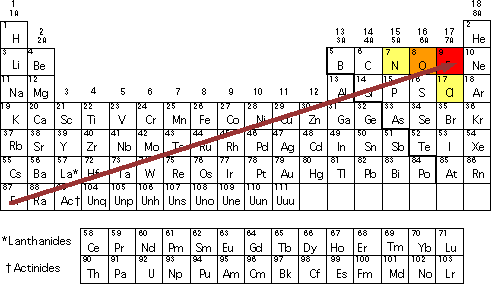
The electronegativity of an element is a measure of the element's ability to attract the electrons which are in a bond.
Earlier we said the fluorine in CF4 has and oxidation number of -1. Fluorine is assinged the oxidation number of -1 because it attracts the electrons in the bond more strongly than the carbon does. Thus, fluorine appears to have an extra electron, -1 oxidation number.
F is the most electronegative element on the periodic table. Followed by O, then N and Cl.
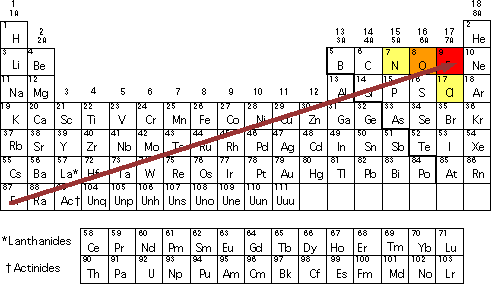
In general the electronegativity of an element increases as one goes up a family.
electonegativity increases in the order: I < Br < Cl < F.
Also, electronegativity increases as one goes across the table.
electonegativity increases in the order: B < C < N < O < F.
When determining oxidation numbers the element with the higher electronegativity wins the electron tug-of-war; so, it is assumed to have complete ownership of the electron for the purpose (porpoise?) of determining oxidation numbers.