Hydrogen-Bonds
Hydrogen bonds are a special case of dipole-dipole interactions. H-bonds are the strongest intermolecular force. (They are worth ca. 5 kJ•mol-1).
Before describing an H-bond let's establish the components that make up an H-bond.
Two things are needed for hydrogen bonds
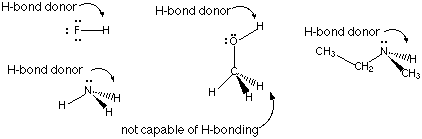
A hydrogen bond donor is a hydrogen atom that is covalently bonded to a highly electronegative atom; N, O, or F.A hydrogen bond acceptor is a highly electronegative atom such as N, O, or F.
H-bond donor examples

H-bond acceptor examples
