|
|
|
|
|
|
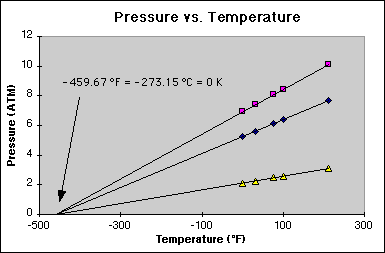
0.0 |
5.2 |
6.9 |
2.1 |
|
32.0 |
5.6 |
7.4 |
2.3 |
|
77.0 |
6.1 |
8.1 |
2.5 |
|
100.0 |
6.4 |
8.4 |
2.6 |
|
212.0 |
7.7 |
10.1 |
3.1 |