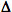 Hf° is the change in
enthalpy associated with the formation of a compound from the
elements. Everything is in its standard
state
Hf° is the change in
enthalpy associated with the formation of a compound from the
elements. Everything is in its standard
stateStandard enthalpies of formation.
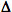 Hf° is the change in
enthalpy associated with the formation of a compound from the
elements. Everything is in its standard
state
Hf° is the change in
enthalpy associated with the formation of a compound from the
elements. Everything is in its standard
state
The heat of formation of NO2 is
From a table of 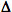 Hf°
values reactions can be put together using Hess's Law.
Hf°
values reactions can be put together using Hess's Law.
Determine the 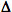 H for the following
reaction
H for the following
reaction
Construct each compound from its elements and the
sum the 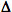 Hf
°.
Hf
°.
The reactions are...
Use the 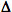 Hf ° reactions
to make the reaction of interest.
Hf ° reactions
to make the reaction of interest.
Since MgO is a reactant the MgO formtation reaction must be turned around. Since the reaction is turned around the DH must also be turned around; i.e., the additive inverse is used.
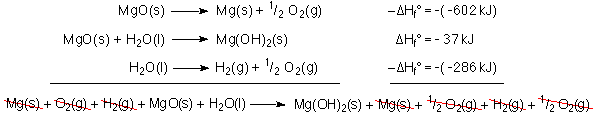
![]()
You may have noticed that we do not have to write down all of the reactions.
You may have noticed that the 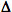 Hf°
of the reactants is always subtracted from the
Hf°
of the reactants is always subtracted from the 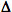 Hf°
of the products.
Hf°
of the products.
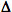 H =
H =
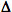 Hf°prod
-
Hf°prod
- 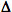 Hf°reactants
Hf°reactants
Calculate 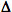 H for the following reaction
using
H for the following reaction
using 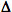 Hf ° values.
Hf ° values.
construct each compound from its elements and the
sum the 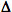 Hf°'s
Hf°'s
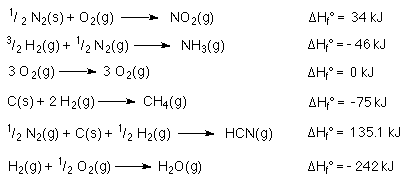
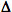 H =
H = 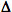 Hf°prod
-
Hf°prod
- 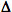 Hf°reactants
Hf°reactants
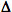 H = 6(-242 kJ) + 2(135.2 kJ) -
2(-75 kJ) - 3(0 kJ) - 2(-46 kJ) = -940 kJ
H = 6(-242 kJ) + 2(135.2 kJ) -
2(-75 kJ) - 3(0 kJ) - 2(-46 kJ) = -940 kJ